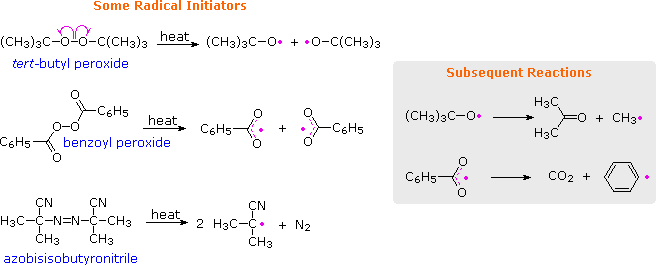
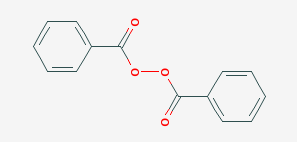
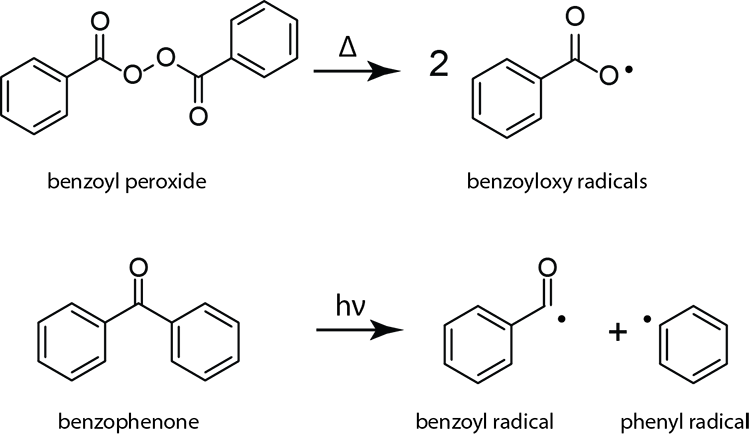
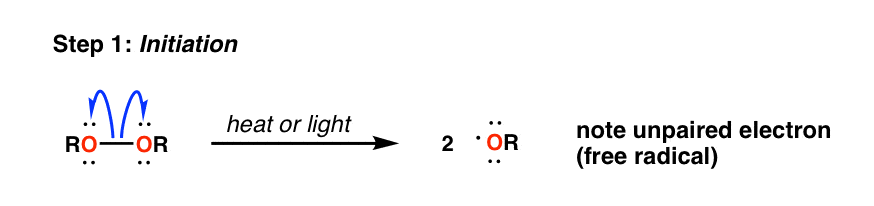
Peresters are the peroxy analog of esters and have general structure RC(O)OOR The O−O bond of peroxides easily breaks, producing free radicals of the form RO • (the dot represents an unpaired electron) Thus, organic peroxides are useful as initiators for some types of polymerisation, such as the epoxy resins used in glassreinforced plasticsThese free radicals are usually produced by decomposing a peroxide such as ditertbutyl peroxide or benzoyl peroxide, shown below In the presence of either heat or light, these peroxides decompose to form a pair of free radicals that contain an unpaired electronFree radical mechanism or by an excited state mechanism132 Typically, photochemically labile compounds called sensitizers are added Some examples of photosensitizers are anthracene, tbutyl peroxide, benzoyl peroxide, 1hydrocycyclohexyl phenyl ketone, and azoisopropane Figure 26 shows examples of various photoinitiators The mechanism

Thermal Decompositions Of A Benzoyl Peroxide B Aibn And C Download Scientific Diagram
Free radical benzoyl peroxide structure
Free radical benzoyl peroxide structure-Search results for free radical at SigmaAldrich Changes will be taking place on SigmaAldrichcom on June 5, 21 that include visual and functional updatesIn the presence of benzoyl peroxide, the addition of HBr to propene involves free radical mechanism in which Brfree radical is obtained by the action of benzoyl peroxide on HBr (i) Br radical adds to propene to form the more stable 2° free radical (ii) Free radical thus obtained rapidly abstracts a hydrogen atom from HBr to form 1bromopropane


New Copper I Complex Based Initiating Systems In Redox Polymerization And Comparison With The Amine Benzoyl Peroxide Reference Polymer Chemistry Rsc Publishing
Polymer , 1 , Fluorinated derivatives of benzoyl peroxide as initiators of radical polymerizations The effect of the chemical structure of chain ends on the thermal degradation of polystyreneThe course of the reactions of benzoyl peroxide with cyclohexane and cyclohexene may be accounted for by a mechanism involving free radicals and radical chain reactions With cyclohexane, the average length of the chain hardly exceeds one step and one mole of hydrocarbon reacts with one mole of peroxideThis synthesis used benzoyl peroxide as a free radical initiator to polymerize styrene monomer First, the benzoyl peroxide must be split to become a peroxy radical The chain is then initiated when a peroxy radical combines with a styrene monomer, forming an active center on the end opposite the initiator
Benzoyl peroxide (BPO) Dicumyl peroxide (DCP) Methyl ethyl ketone peroxide (MEKP), Cumene hydroperoxide (CHP) Upon heating, these peroxides dissociate to form two radicals which attack the monomer double bonds and add to them (addition) This forms a reactive radical center which can propagate to form a polymerDownload Free PDF Reactivity of Benzoyl Peroxide/Amine System as an Initiator for the Free Radical Polymerization of Dental and Orthopaedic Dimethacrylate Monomers Effect of the Amine and Monomer Chemical StructureFree radical bromination in order to verify the relative reactivities of various types of C–H bonds benzoyl peroxide (MW 2422) and 25 mL chlorobenzene (25 mL) to the vial The structure of the product should be drawn on the NMR and each of
Benzoyl peroxide C14H10O4 CID 7187 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and moreThe structure of these radicals is shown in the figure below, along with the notation used to denote them Note the difference between hydroxyl radical and hydroxyl ion, which is not a radical Another radical derived from oxygen is singlet oxygen, designated as 1 O 2Define benzoyl peroxide benzoyl peroxide synonyms, benzoyl peroxide pronunciation, benzoyl peroxide translation, English dictionary definition of benzoyl peroxide n A flammable white granular solid, C14H10O4, used as a bleaching agent for flour and lipids, as a drying agent, as a polymerization catalyst, and in


Non Ionic Chemical Reactions



Free Radical Polymerisation Requires A Free Radical Initiator The Most Commonly Used Free Radical Initiator Is
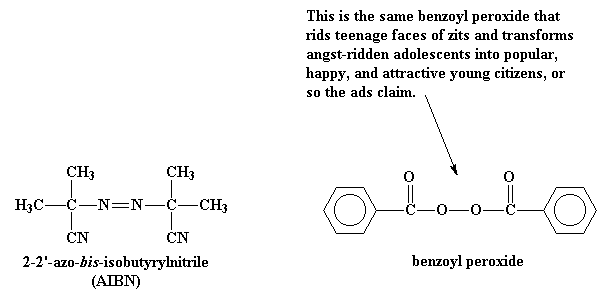
Peroxide (hydrogen peroxide) hydroxyl radical; · Reactivity of Benzoyl Peroxide/Amine System as an Initiator for the Free Radical Polymerization of Dental and Orthopaedic Dimethacrylate Monomers Effect of the Amine and Monomer Chemical Structure Sideridou, Irini DIt consists of two benzoyl groups bridged by a peroxide link BPO readily undergoes symmetrical fission (homolysis), forming two benzoyloxy radicals 24 The two fragments with unpaired electrons are called free radical initiators Following its generation, the initiating free radicals react with a monomer unit thereby creating growing polymer chains The reactivity of BPO depends on


16 4 Useful Polymers Formed By Radical Chain Reactions Chemistry Libretexts


Free Radical Vinyl Polymerization
Does bimolecular termination dominate in benzoyl peroxide initiated styrene freeradical polymerization?Benzoyl peroxide is a topical treatment for acne that generates free radicals to break down comedones and increase the rate of epithelial cell turnover 1,3,4,5,9,10,11,12 It has a short duration of action as its active free radical metabolites quickly react to form inactive metabolites 1 The therapeutic index is wide, as overdoses are rare, however patients may still experience skinCommonly, 2,2'Azobis(2methylpropionitrile) and Benzoyl peroxide suit these requirements If the desired polymerization occurs at or below °C, then special, low temperature free radical initiators need to be used



What Is The Role Of Benzoyl Peroxide In Addition Polymerisation Of Alkenes Explain Its


Unit 4 Advanced Organic Chemistry Polymerisation Page
Free radical polymerizations are usually performed using one of four different methods bulk polymerization, solution polymerization, suspension polymerization and emulsion polymerization This experiment illustrates certain aspects of bulk free radical polymerization We shall study suspension polymerization in experiment 13Benzoyl peroxide induced graft polymerization of 2methyl5vinylpyridine onto polyester/wool blend BPO is used with aniline accelerators or where heat is used to cure the resin CHEMICAL SYNTESIS CATALYST It is used as a catalyst in oxidation and sulfonation reactions It is a strong free radical source containing more than 49% of activeNoun 1 benzoyl radical the univalent radical derived from benzoic acid benzoyl group chemical group, radical, group (chemistry) two or more atoms bound together as a single unit and forming part of a molecule Based on WordNet 30, Farlex clipart collection © 0312 Princeton University, Farlex Inc


Polystyrene



How Does Benzoyl Peroxide Work In The Skin Acne Org
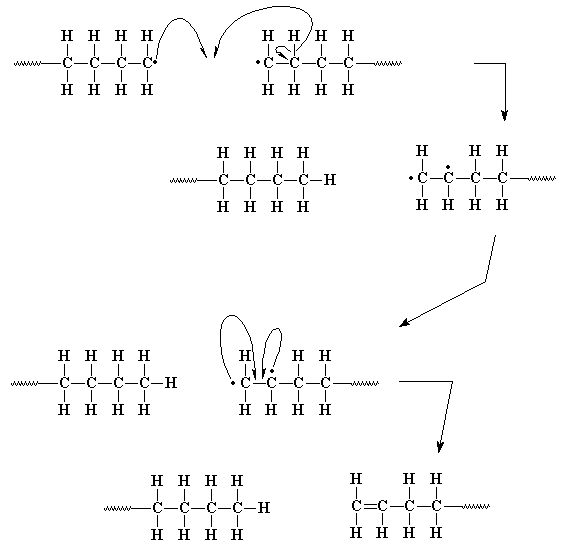
A new method to functionalize multiwalled carbon nanotubes (MWCNT) with 3aminopropyltriethoxysilane (APTES) has been established using benzoyl peroxide (BPO) as initiator The functionalization was carried out through free radical reaction by thermal decomposition of benzoyl peroxide at 110°C The reaction liberated carbon dioxide and generated phenyl free radicalsIn the polymerisation of ethene to polythene Ethene is heating or exposing to light with benzoyl peroxide initiator In this process a larger free radical is form As this radical reacts with another molecule of ethene, another bigger sized readical is formed The repetition of this sequence is known as chain propagating step · Benzoyl peroxide is a suitable initiator for addition polymerization reactions with free radical polymerization techniques Benzoyl peroxide can experience a reaction homolytic becomes radical at temperatures above 70°C The use of benzoyl peroxide in polymerization is very high suitable for polymerization of monomers that have double bonds



Dmpt Activator And Bpo Initiator React To Form Two Free Radicals Download Scientific Diagram


Benzoyl Peroxide 1 Rayeneh Group
Commercial polystyrene is mostly synthesized by bulk, suspension or solution polymerization of ethylbenzene (styrene) The most common method is free radical polymerization, using benzoyl peroxide as initiator However, other initiators such as redox systems and azo compounds can be used as well to start the polymerizationBenzoyl peroxide (BPO) can be used as a coreactant, because, like persulfate, BPO can produce a reactive oxidizing agent, benzoate radical (C 6 H 5 CO 2 •), after it is reduced The newly formed benzoate radical is energetic enough to react with some cation radicals to form the excited states, since the redox potential value for C 6 H 5 CO 2 •/C 6 H 5 CO 2 − couple (> 15 V vs SCE) (144)When composite resins were first introduced, their polymerization involved free radical initiation by a combination of benzoyl peroxide with tertiary amine activator, delivered in two different pastes, known respectively as base and catalyst


Global Radical Initiator Market Briefing Trends Applications Types Research Forecast To 25 The Courier



Mechanism Of Redox Initiation By The Benzoyl Peroxide Amine System 12 Download Scientific Diagram
Uses of Benzoyl peroxide it can be used to test formaldehyde and bravery sterol And it can be used as quality improver of flour, intermediates of fine chemical products, additives of grain and oil, vulcanization agent of xylene silicone rubber and kyle Frubber, catalyst of free radicals halogenating and radical sensitization reactionBenzoyl peroxide catalytic decomposition of carbon tetrachloride in a model system produces trichloromethyl and trichloromethylperoxyl free radicals These radicals are also produced by CCl4 bioactivation in liver and are considered to be responsible for the deleterious effects ofThe initiation kinetics of the free‐radical polymerization of methyl methacrylate at very high monomer conversions was investigated at 80, 100, and 1°C Using a HPLC (high performance liquid chromatography) analytical technique with UV detection, the consumption of both monomer and initiator as well as the formation of reaction products of the latter could be followed


Free Radical Vinyl Polymerization


Non Ionic Chemical Reactions
In this experiment, benzoyl peroxide will be used to initiate a free radical chain polymerization of the monomer, similiar to the polystyrene synthesis A trace of a weak base is added to neutralize any acid present A control, kept at room temperature, will be compared with a sample polymerized at boiling water temperature Page 2The polymerisation process is a freeradical chain reaction, but forming the initial free radical from stable styrene molecules is quite difficult unless extreme conditions are used This is where adding an initiator like benzoyl peroxide comes in (see a video of this on Youtube) · Benzoyl peroxide exists as a colourless solid which has a crystalline structure It has a faint odour which resembles the smell of benzaldehyde C 14 H 10 O 4 is insoluble in water, somewhat soluble in alcohols, and quite soluble in ethers and chloroform


Benzoyl Peroxide 94 36 0



Pdf Reactivity Of Benzoyl Peroxide Amine System As An Initiator For The Free Radical Polymerization Of Dental And Orthopaedic Dimethacrylate Monomers Effect Of The Amine And Monomer Chemical Structure Dimitris Achilias And
Get ready to look at this polymerization for a long time D________________________________________000 Dry benzoyl peroxide demonstration023 Dry benzoylCharacter of medium (Fig 1) As far as benzoyl peroxide is in solid state the existence of decomposition reaction is not observed under given experimental conditions This case can be demonstrated in a differential enthalpic curve for the mixture of benzoyl peroxide with anthracene (curve 2) as well as for pure benzoyl peroxide (curve 1) in theBenzyl peroxide C14H14O2 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities



Benzoyl Peroxide An Overview Sciencedirect Topics



Benzoyl Peroxide An Overview Sciencedirect Topics
0425 · Benzoyl peroxide produces free radicals, and therefore some concern exists that this might lead to skin aging However, benzoyl radicals may also reduce inflammation, which could reduce skin aging To be safe, apply an antioxidantcontaining moisturizer after treating with benzoyl peroxideThe free radical homopolymerization of BisphenolAbis(glycidyl methacrylate) (BisGMA), mine redox couple was studied by differential scanning calorimetry As amine, parasubstituted N,Ndimethylaniline derivatives were usedBenzoyl peroxide has been linked to cancer for a number of years and many research journal entries state "benzoyl peroxide is a free radicalgenerating skin tumor promoting agent" Performing a word search of the words "benzoyl peroxide cancer" in PubMed in the National Library of Medicine produces 102 articles from medical publications dealing with research aspects of benzoyl peroxide
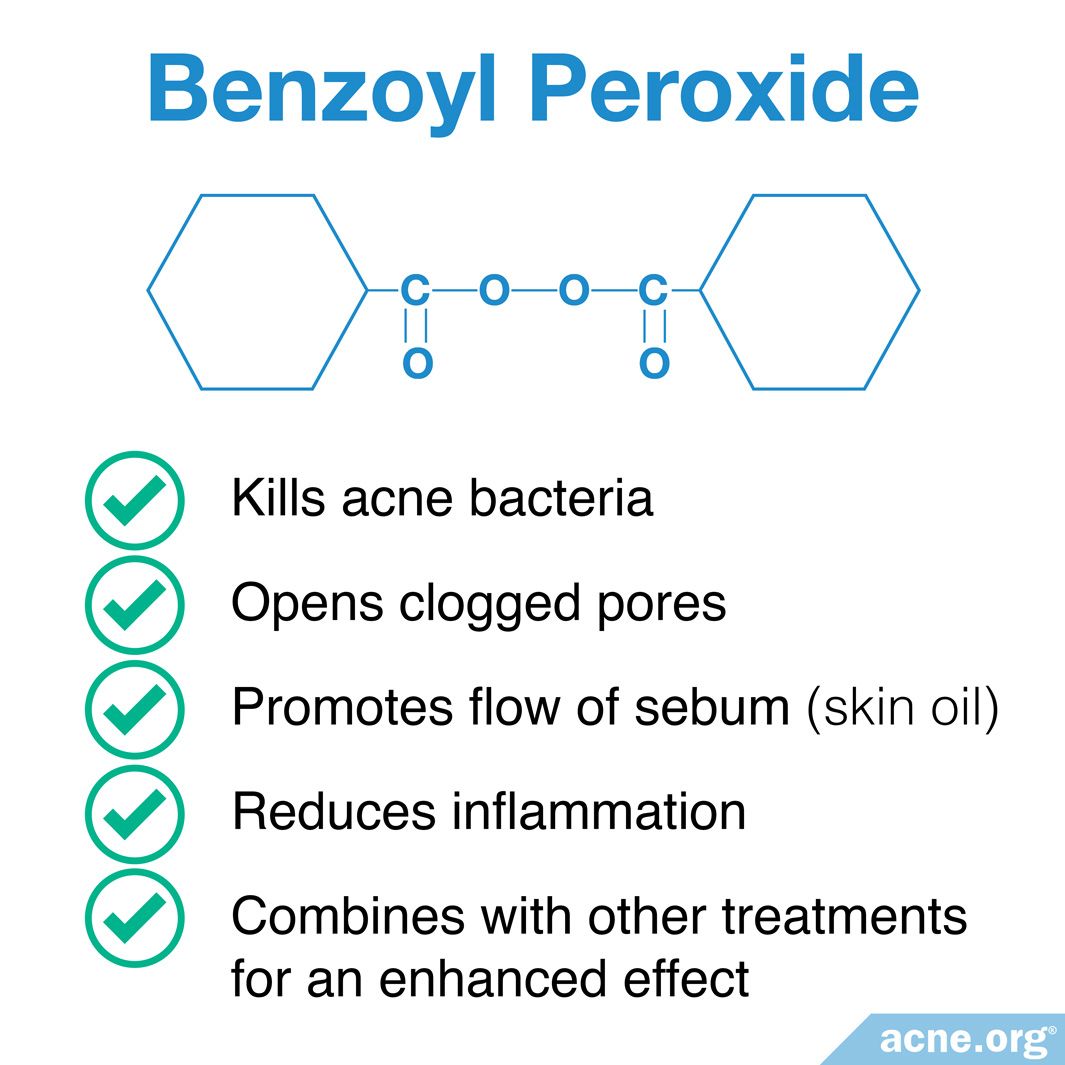


How Does Benzoyl Peroxide Work In The Skin Acne Org



Uv Curing Part Three Free Radical Photoinitiators Polymer Innovation Blog
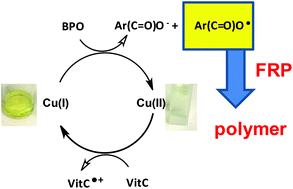
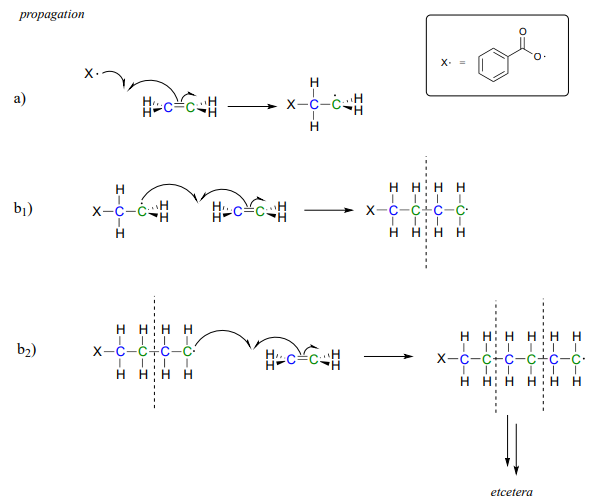
In this chapter, we only discuss free radical chain polymerization The ionic chain polymerization will be discussed in Chap 8 Table 72 lists some common free radical chaingrowth polymers and their uses The chaingrowth polymers count more than 80 % total usage of polymers in our daily life 71 Effect of Chemical Structure of MonomerA radical initiator such as benzoyl peroxide undergoes homolytic cleavage when subjected to high temperatures In the propagation phase, the benzoyl radical (X• in the figure below) adds to the double bond of ethylene, generating a new organic radicalNovel copper complex based initiating systems for redox free radical polymerization (FRP) of methacrylate resins under mild conditions are proposed Remarkably, the FRP performance of the Cu(i)/ascorbic acid (Vitamin C – VitC)/benzoyl peroxide (BPO) redox system is noticeably more significant than that of th
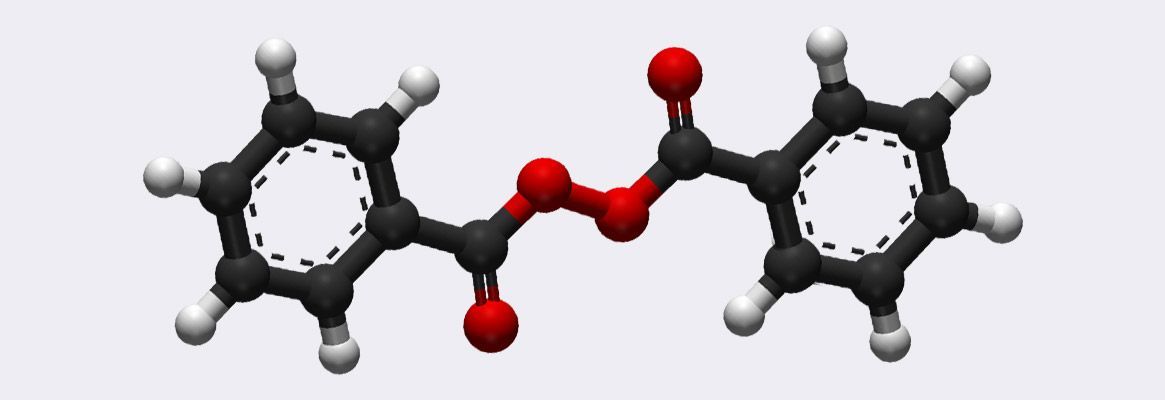


Benzoyl Peroxide Fastness In Home Textiles Structure Of Benzoyl Peroxide Fibre2fashion



What Is The Role Of Benzoyl Peroxide In Addition Polymerisation Of Alkenes Explain Its Mode Of Youtube
Alphatocopherol amplifies benzoyl peroxide free radical decomposition in a chemical system Rota C (1), Tomasi A, Iannone A (1)Department of Biomedical Sciences, University of Modena and Reggio Emilia, Modena, Italy Benzoyl peroxide is commonly used in the treatment of acne, even though some adverse effects have been reported, probably mediated
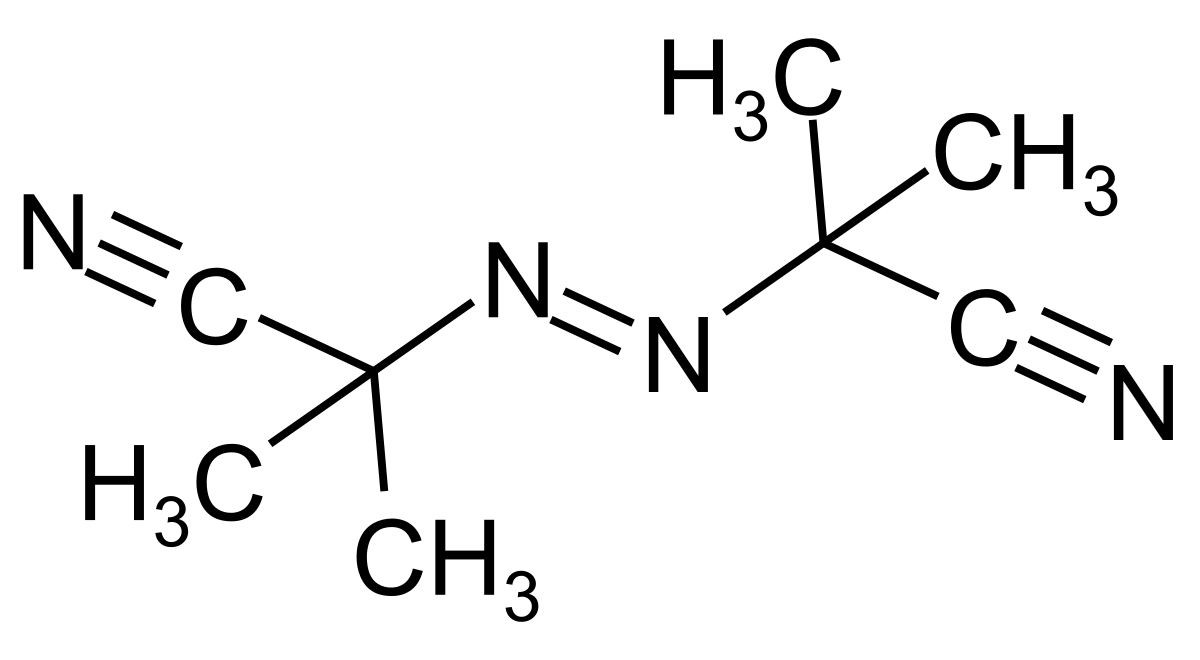


Azobisisobutyronitrile Wikipedia



Pdf Reactivity Of Benzoyl Peroxide Amine System As An Initiator For The Free Radical Polymerization Of Dental And Orthopaedic Dimethacrylate Monomers Effect Of The Amine And Monomer Chemical Structure Dimitris Achilias And



18 4 Radical Reactions In Practice Organic Chemistry Ii


Illustrated Glossary Of Organic Chemistry Benzoyl Peroxide



Chapter 6 Free Radical Polymerization Ppt Download



Free Radical Vinyl Polymerization
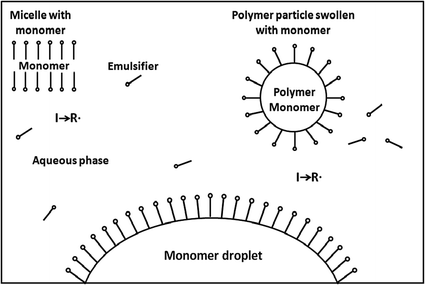


Radical Chain Polymerization Springerlink



Introduction To Polymers 4 3 1 Initiation Openlearn Open University T8 1



Radical Initiator Wikipedia


Benzoyl Peroxide C14h10o4 Chemspider



Learn About Polymerization Of Ethylene Chegg Com


Benzoyl Peroxide Molecule Of The Month February 17 Html Version


Polymerization Reactions
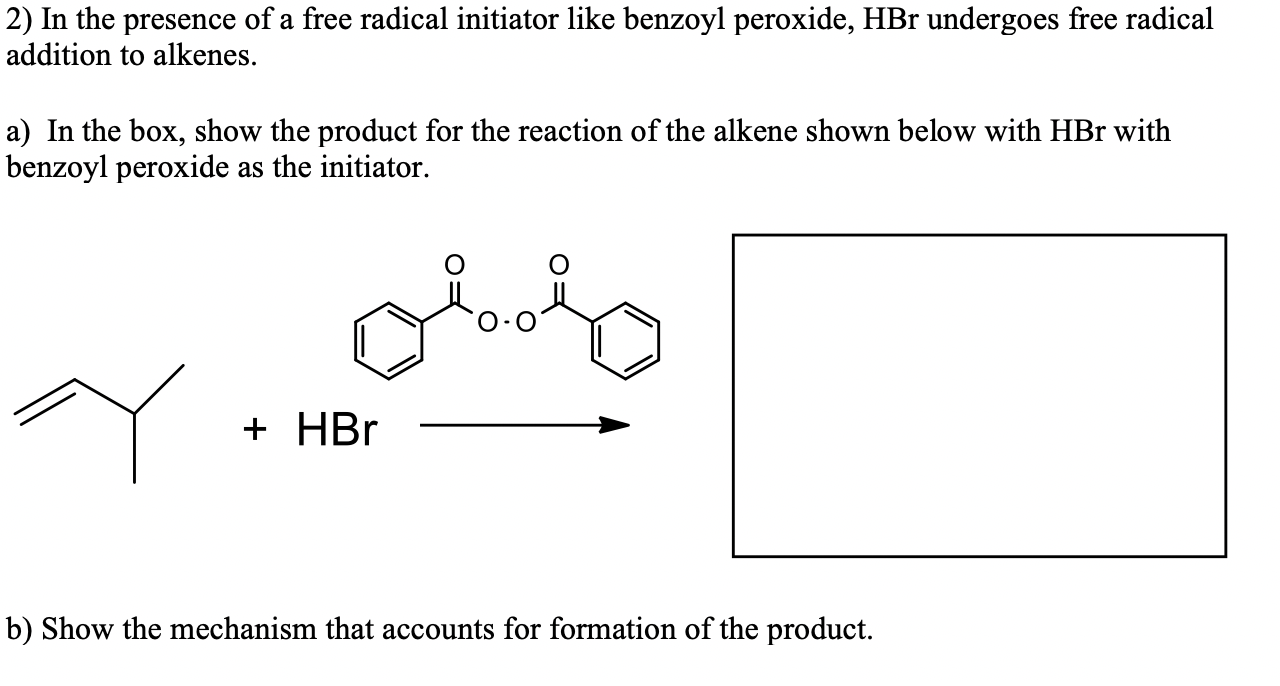


Solved 2 In The Presence Of A Free Radical Initiator Lik Chegg Com
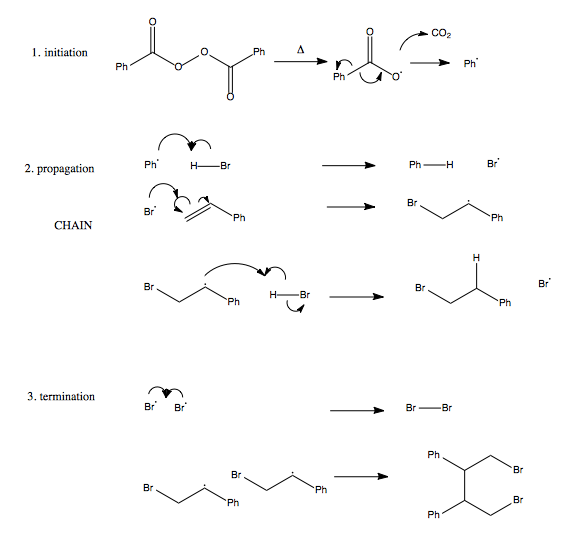


Organic Chemistry 22 Radicals Alkene Halogenation Polymerization



Free Radical Polymerization Animation Iqog Csic Youtube


G Ray Induced Polymerization



Benzoyl Peroxide Podcast Chemistry World
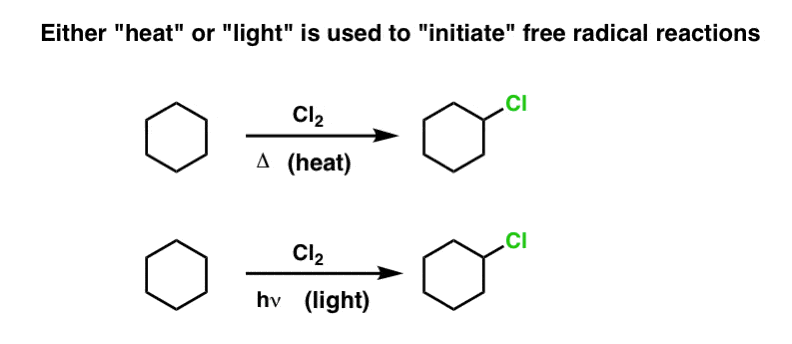


Free Radical Initiation Why Is Light Or Heat Required



Origins And Development Of Initiation Of Free Radical Polymerization Processes



Benzoyl Peroxide


Benzoyl Peroxide Molecule Of The Month February 17 Html Version


Is Benzoyl Peroxide The Same As Hydrogen Peroxide Quora
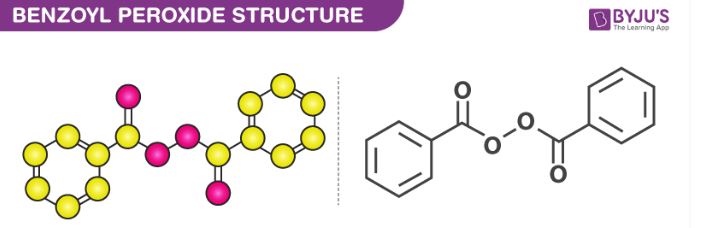


Benzoyl Peroxide Formula Structure Properties And Uses



Write The Name And Structure Of One Of The Common Initiators Used In Free Radical Addition Polymerisation
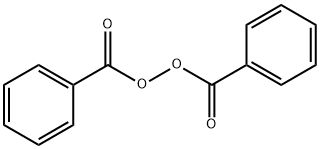


Benzoyl Peroxide 94 36 0



New Copper I Complex Based Initiating Systems In Redox Polymerization And Comparison With The Amine Benzoyl Peroxide Reference Polymer Chemistry Rsc Publishing


Polystyrene
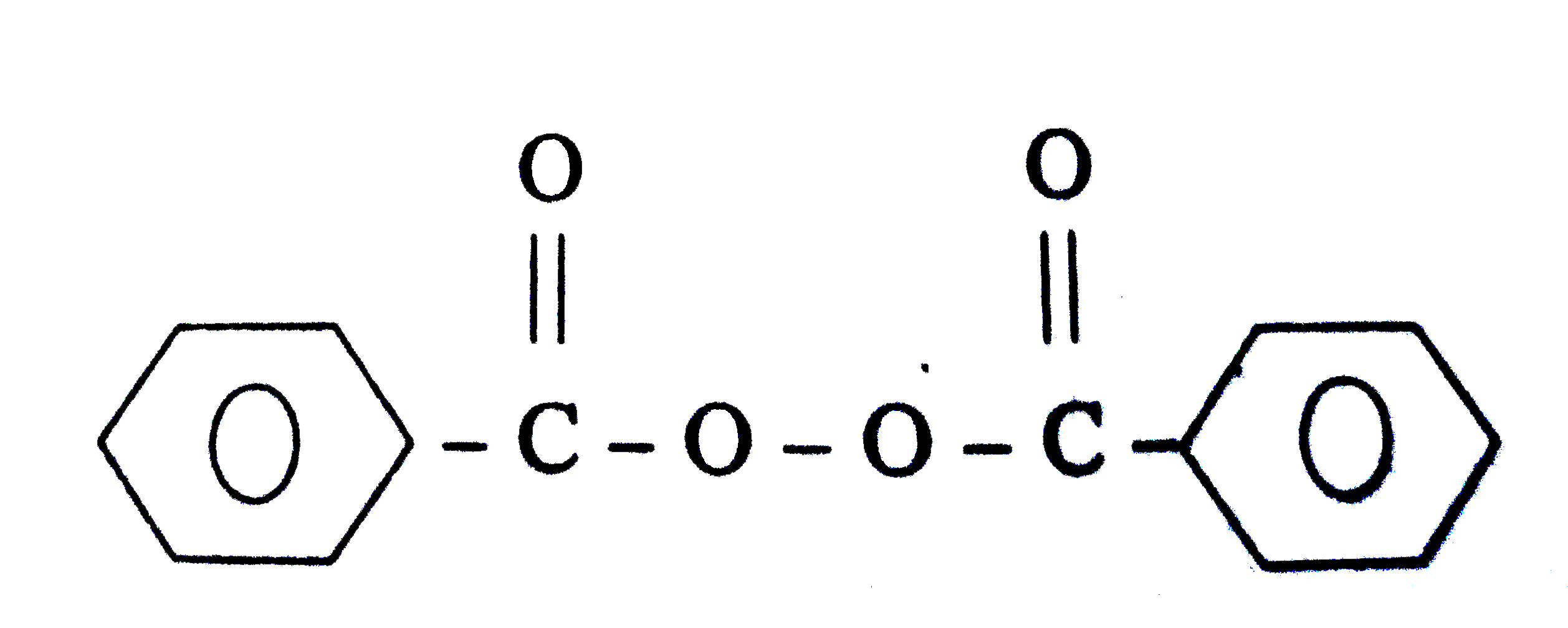


Write The Name And Structure Of One Of The Common Initiators Us
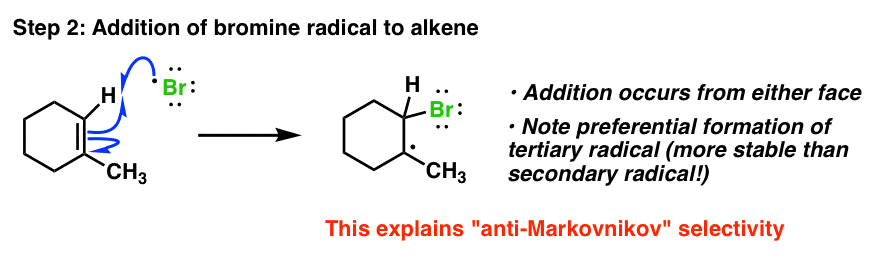


Radical Addition Of Hbr To Alkenes W Roor Peroxides Mechanism
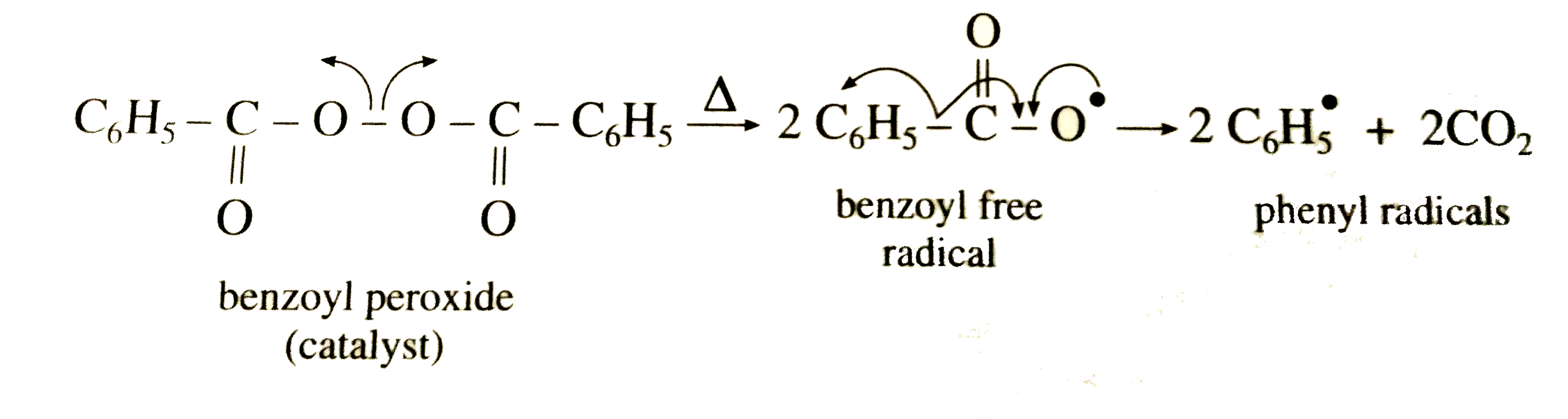


Write The Steps Involved In The Free Radical Mechanism In Polym



Structure



Benzoyl Peroxide


Polymers One Important Class Of Organic Structure We Do Not Really Have Time To Cover In Class Is Polymers Polymers Have Obviously Transformed The Material Aspects Of Our Lives In The Last 75 Years Or So The Study Of Polymer Chemistry And Physics Is A Huge



A Dibenzoyl Peroxide Commonly Known As Benzoyl Peroxide Or Bpo Download Scientific Diagram


Unit 5 Radicals And Radical Reactions



A Polyethylene Exists Either As A Linear Straight Chain Polymer Or As A Branched Polymer Which Is The High Density Form Explain Socratic
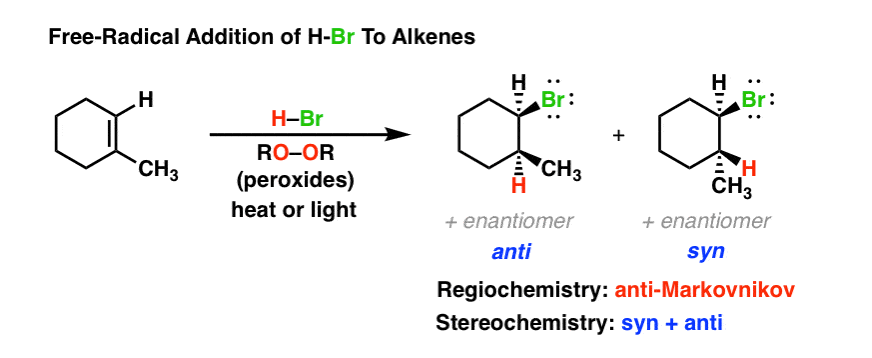


Radical Addition Of Hbr To Alkenes W Roor Peroxides Mechanism
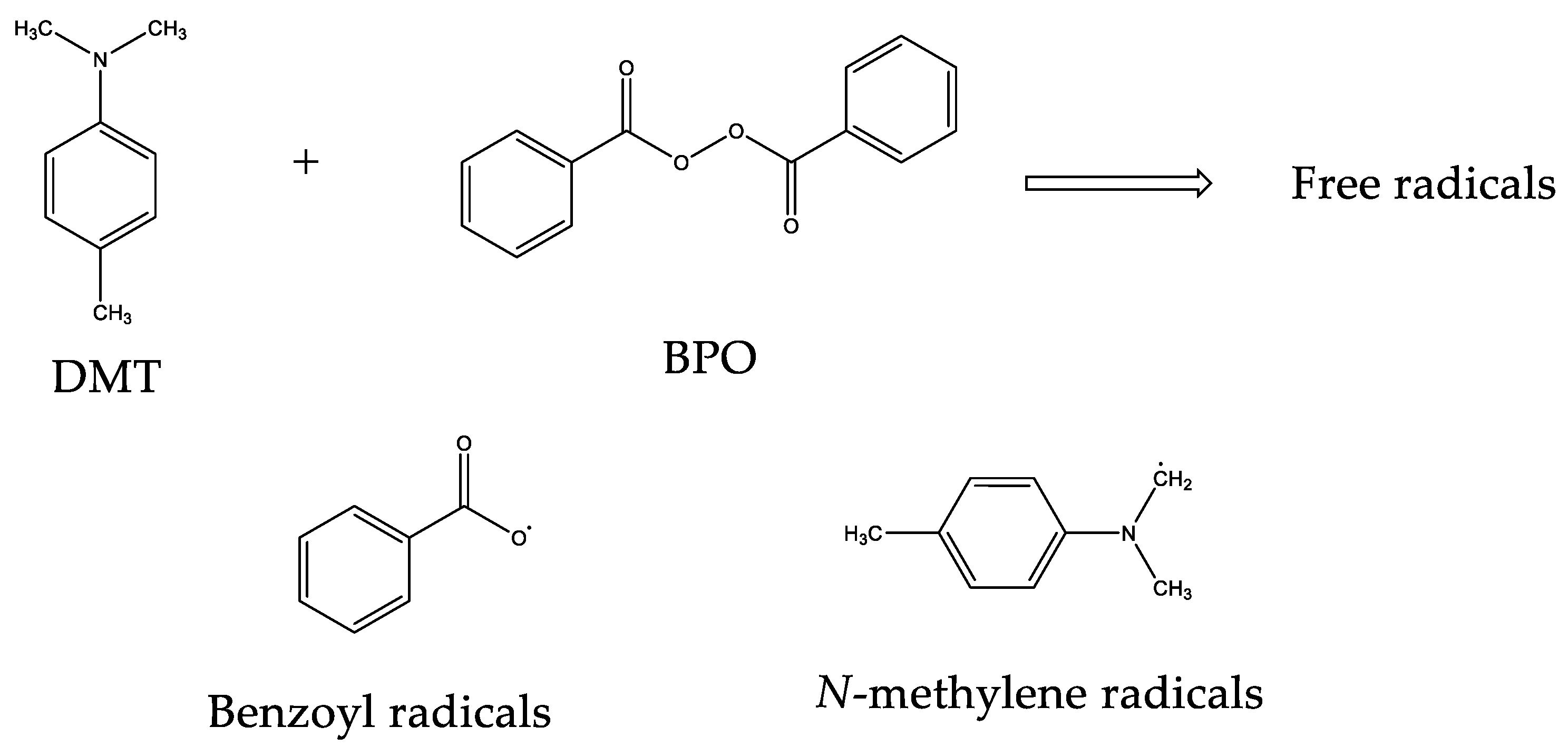


Polymers Free Full Text Thermal And Mechanical Characterization Of Ema Tegdma Mixtures For Cosmetic Applications Html



Write The Name And Structure Of One Of The Common Initiators Used In Free Radical Addition


Radical Addition Of Hbr To Alkenes W Roor Peroxides Mechanism
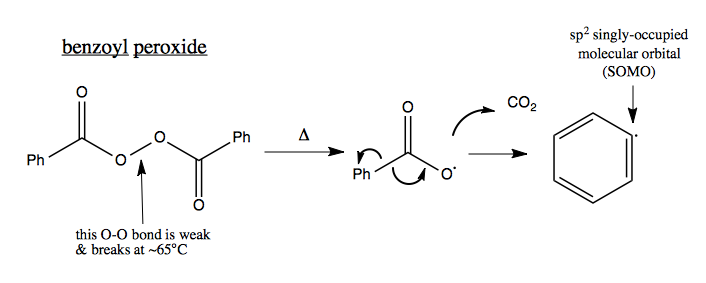


Organic Chemistry 22 Radicals Alkene Halogenation Polymerization


Illustrated Glossary Of Organic Chemistry Benzoyl Peroxide


Polystyrene
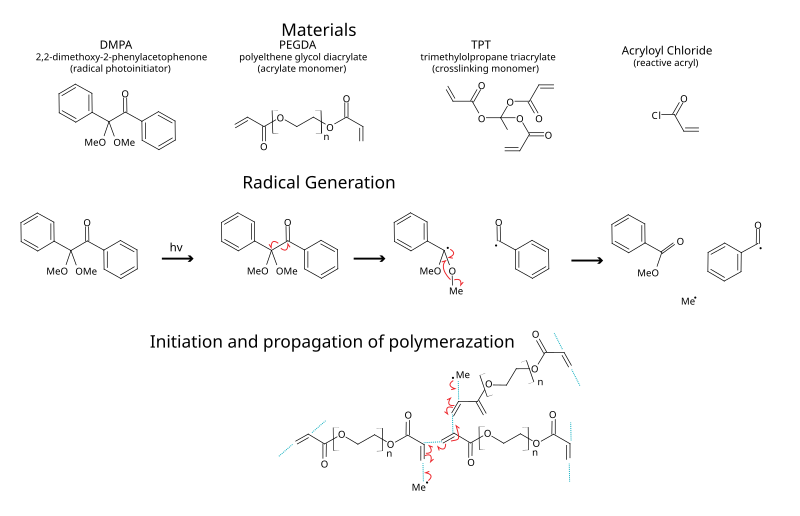


Photoinitiator Wikipedia



Chapter 6 Free Radical Polymerization Ppt Download



Luperox 5 Benzoyl Peroxide Aldrich Cas No 94 36 0 Sigma Aldrich



Write The Free Radical Mechanism For The Polymerization Of Ethene From Chemistry Polymers Class 12 Tripura Board
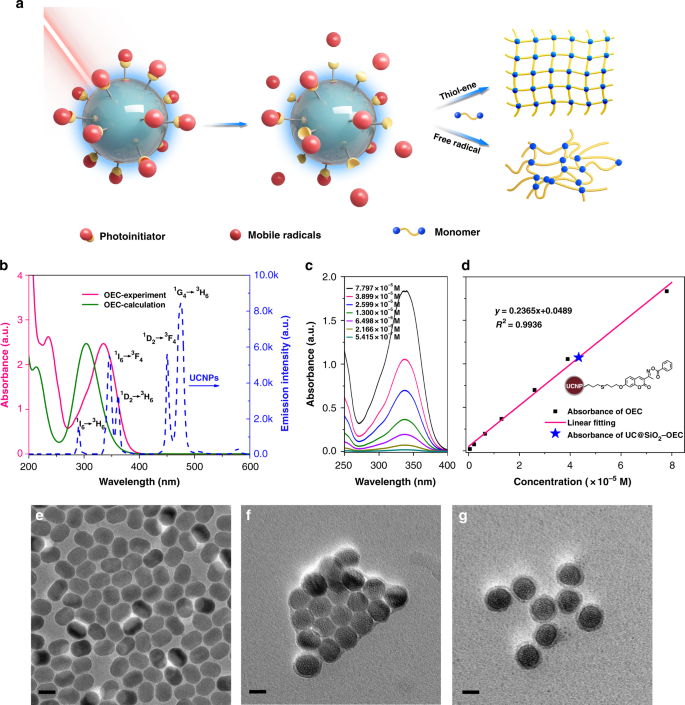


Highly Efficient Dandelion Like Near Infrared Light Photoinitiator For Free Radical And Thiol Ene Photopolymerizations Nature Communications



Azobisisobutyronitrile Wikipedia



Solved Consider The Benzoyl Peroxide Initiated Polymeriza Chegg Com
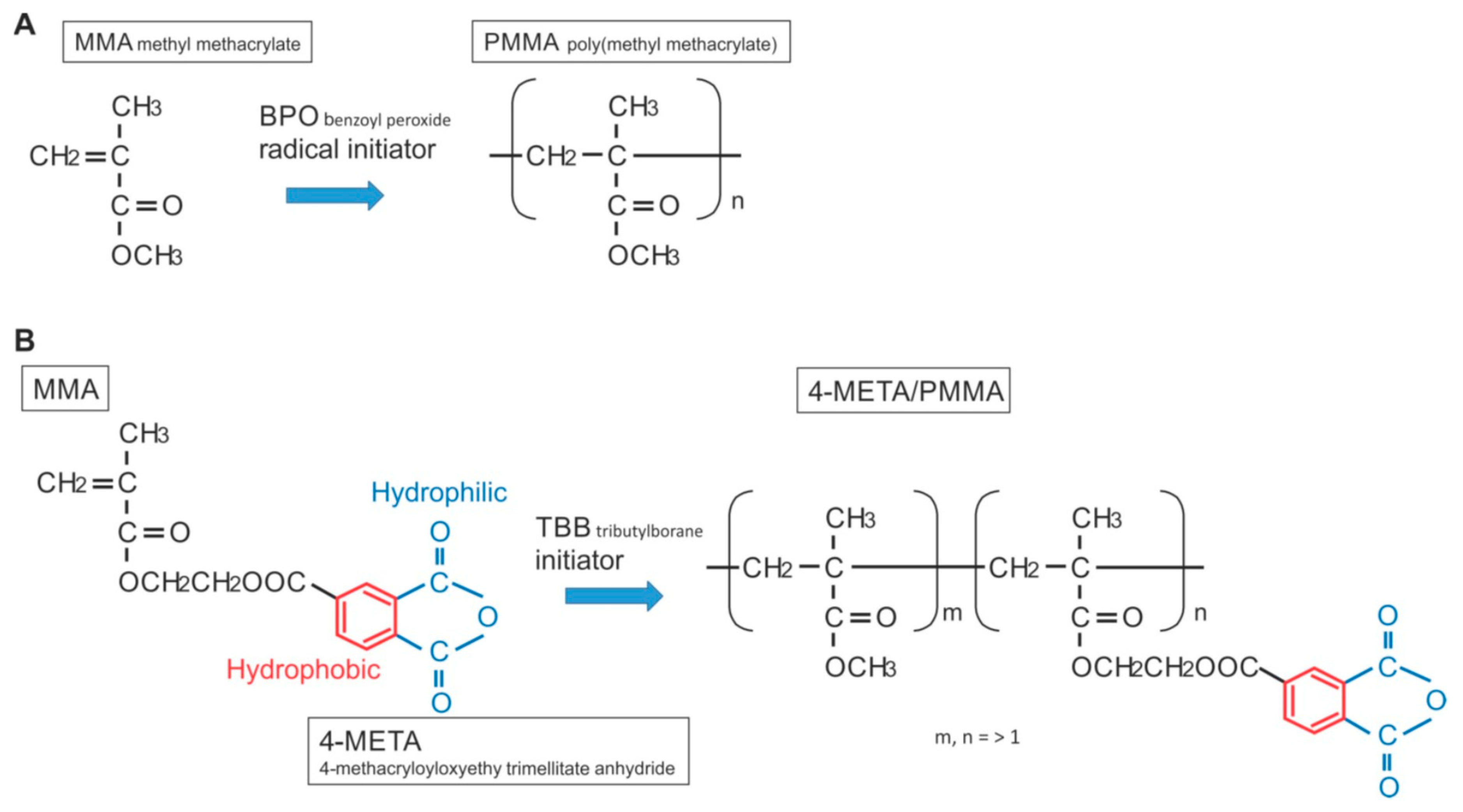


Ijms Free Full Text Novel Osteogenic Behaviors Around Hydrophilic And Radical Free 4 Meta Mma Tbb Implications Of An Osseointegrating Bone Cement Html
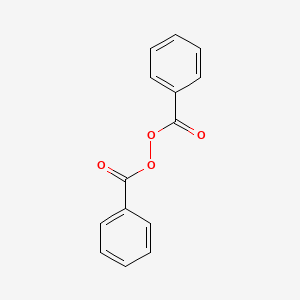


Benzoyl Peroxide C14h10o4 Pubchem


Benzoyl Peroxide Molecule Of The Month February 17 Html Version


Polymers Experiment 1



Chapter 14 15 Polymer Studies Ppt Video Online Download


Polystyrene



Benzoyl Peroxide An Overview Sciencedirect Topics



Free Radical Vinyl Polymerization Polymerization Radical Chemistry
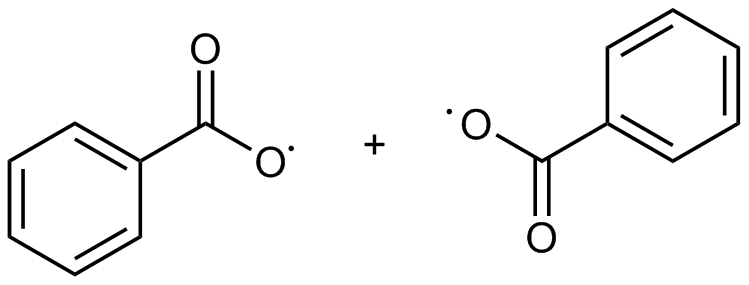


What Does Benzoyl Peroxide Do Lab Muffin Beauty Science



Benzoyl Peroxide Wikipedia
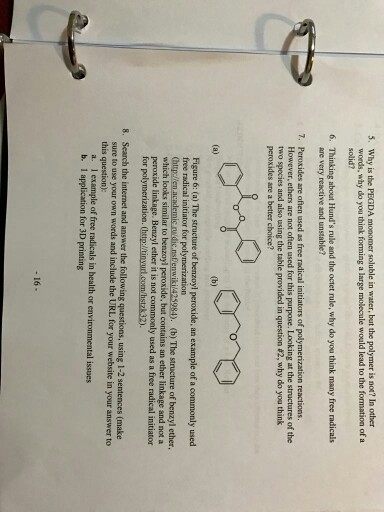


Solved 5 Why Is The Pegda Monomer Soluble In Water But Chegg Com
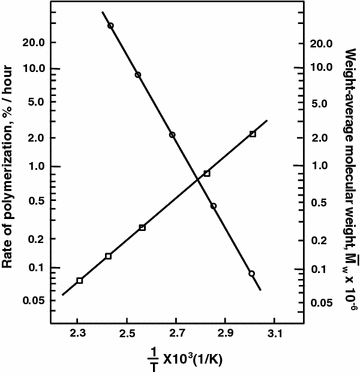


Radical Chain Polymerization Springerlink
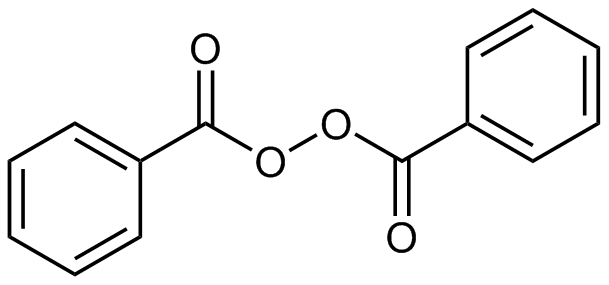


What Does Benzoyl Peroxide Do Lab Muffin Beauty Science



Experiment 16



Thermal Decompositions Of A Benzoyl Peroxide B Aibn And C Download Scientific Diagram



Does Bimolecular Termination Dominate In Benzoyl Peroxide Initiated Styrene Free Radical Polymerization Sciencedirect



Thermal Hazard Evaluation Of The Autocatalytic Reaction Of Benzoyl Peroxide Using Dsc And Tam Iii Sciencedirect



Benzoyl Peroxide An Overview Sciencedirect Topics
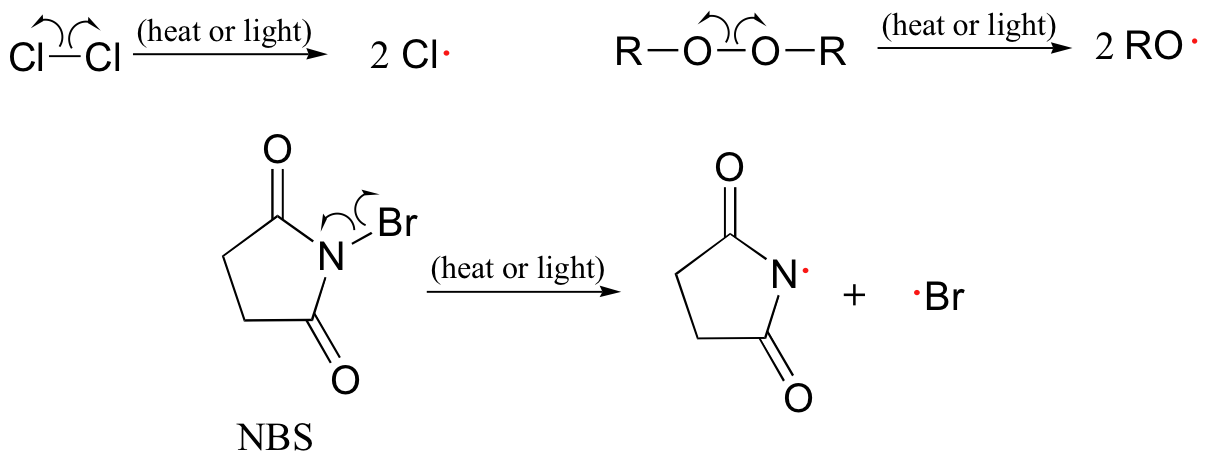


11 5 Radical Chain Reactions Chemistry Libretexts



Figure 1 From Biointerfaceresearch Com Bone Cement A Review Semantic Scholar



Give The Structures Of The Free Radical Intermediates In The Peroxide Initiated Reaction Of Hbr With The Following Homeworklib
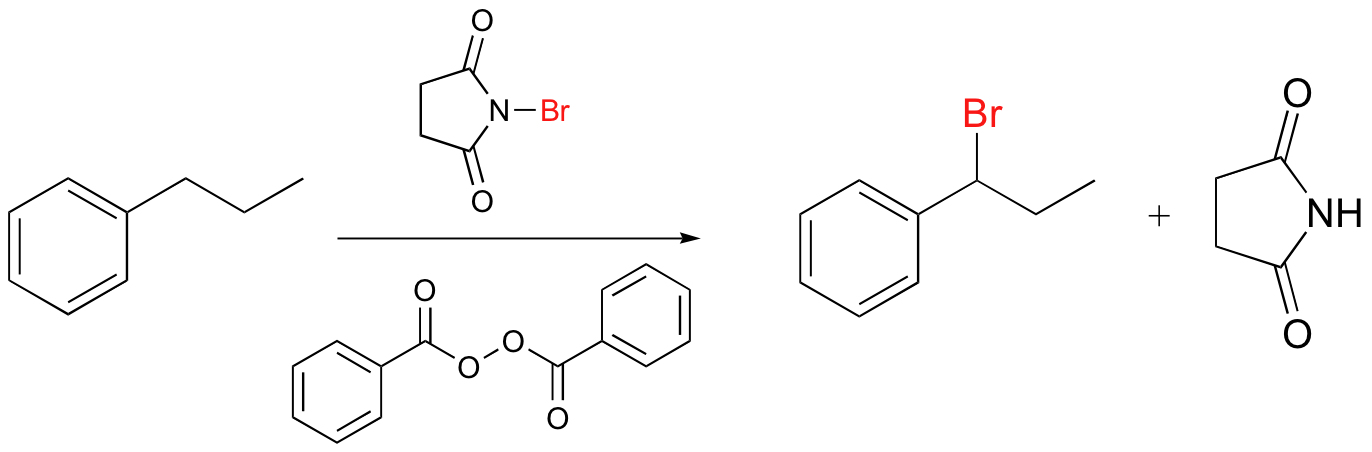


11 5 Radical Chain Reactions Chemistry Libretexts



Polymerization Reactions Chapter 4 Fall Outline Introduction Classifications Chain Polymerization Free Radical Initiation Reaction Mechanism Ppt Download



Novel N Acyloxytrialkylammonium Salts As Initiators For Free Radical Polymerization Of Methacrylates Journal Of Materials Chemistry Rsc Publishing Doi 10 1039 b



Free Radical Polymerisation Requires A Free Radical Initiator The Most Commonly Used Free Radical Initiator Is


